Sort
Categories
- #EMSPVirtual2021
- Associate members
- Document
- EMSP Events
- EMSP Events|EMSP News
- EMSP News
- EMSP News|From Europe
- EMSP News|From Europe|Stakeholder Events
- EMSP News|MS Research
- EMSP News|Press Releases
- EMSP News|Young People with MS
- From Europe
- From Europe|Member News
- From Europe|Stakeholder Events
- From Europe|Young People with MS
- Full members
- Full members|Member News
- Member News
- Member News|Young People with MS
- MS Research
- News – EMSP
- News2
- Press Releases
- Project Updates
- Stakeholder Events
- Uncategorized
- Young People with MS
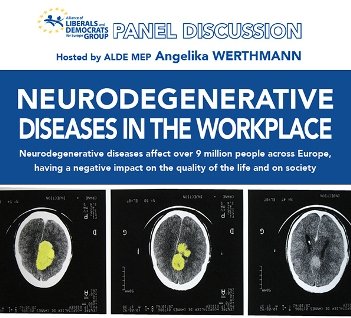
Pledge for a Written Declaration in support of people with NDDs
Member of the European Parliament Angelika Werthmann brought a fresh perspective towards improving the quality of life of over 9…
ECTRIMS 2013: MS in the Workplace, high on the agenda of European patients’ organisations
A number of 26 patients’ organisations from across Europe, including the European Multiple Sclerosis Platform (EMSP), came together to discuss…
MS Nurse PROfessional launched in Ireland
The MS Nurse PROfessional online training programme for MS nurses has expanded its coverage to include Ireland. The national launch…
MS Nurse PROfessional launched in Ireland
The MS Nurse PROfessional online training programme for MS nurses has expanded its coverage to include Ireland. The national launch…
“Hopeful times” anticipated by leading MS advocate upon approval of MS treatment
The European Commission has granted marketing authorization for Aubagio 14 mg (teriflunomide), an oral therapy recommended for the treatment of…
The Written Declaration bid for neurodegenerative diseases: a useful experience
The bid for a Written Declaration for neurodegenerative diseases (NDDs) in the workplace managed to gather 161 signatures from Members…
 Your Account
Your Account