
WISDOM
Well-being improvement through the Integration of healthcare and research Data and models with Out border for chronic iMmune-mediated diseases
Summary
Enabling integration of medical and research data, secure data sharing and leveraging responsible state-of-the-art artificial intelligence (AI)-mediated models opens immense possibilities to mitigate the impact of chronic immune mediated diseases (CIMDs) affecting 10% of Europeans. The consortium’s overarching aim is to convert complex biological information from the existing data sources into actionable insights. WISDOM builds on the premise that computational tools can provide valuable knowledge and guide decision-making at critical stages in the individual patient journey, from diagnosis to treatment initiation and optimisation. To unlock the potential of the existing data, WISDOM will address barriers of data integration and accessibility and deploy novel approaches for data processing, harmonisation, integration, and secure, trustworthy data sharing with federated access.
WISDOM’s approach and impact. By the development and validation of risk stratification, disease outcome prediction and personalised intervention tools, WISDOM aims to expedite the identification and diagnostic follow-up of individuals at high risk of developing disease, and more accurate treatment installation, monitoring and change as well as advice regarding lifestyle changes.
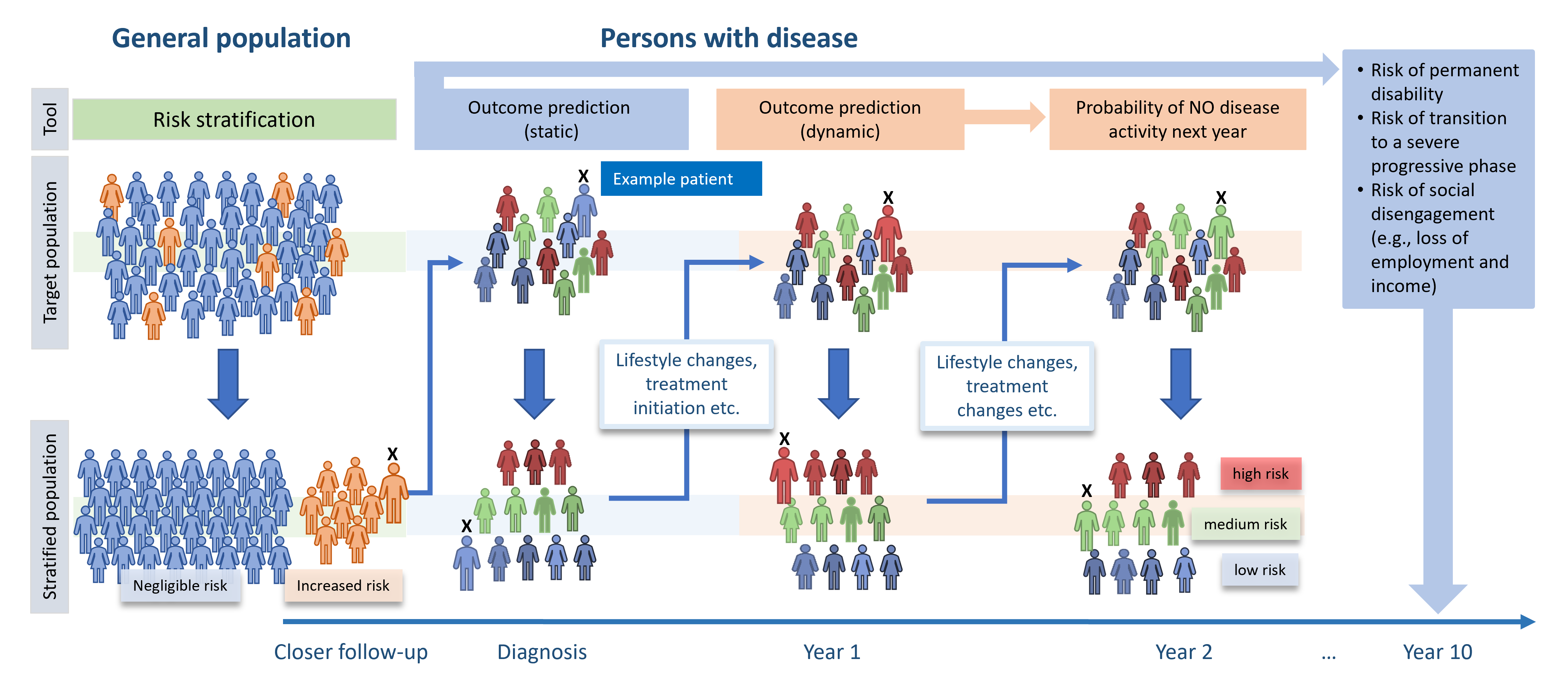
Objectives:
- Develop and apply tools for harmonising, integrating, and sharing real-world health and research data to release the potential of more comprehensive information for better management of CIMDs.
- Develop and apply data access and analytical tools enabling secure, cost-effective, and trustworthy sharing of clinical, registry and research data across diseases, medical areas, and national borders.
- Develop computational risk stratification and outcome prediction models and tools in different use cases to advance our understanding of risk factors and management of CIMDs throughout disease trajectories.
- Prospectively validate the models and clinical tools on technical, clinical and user aspects to facilitate data-driven and patient-focused diagnosis, treatment and monitoring towards better patient outcomes and promotion of well-being in general.
Value for patients:
The project efforts will lead to better patient outcomes, reduced healthcare costs, and increased trust in the use of health data for research and innovation, ultimately benefiting patients, healthcare providers, and society. Additionally, by improving treatment precision, WISDOM seeks to improve patient outcomes, leading to a better quality of life for patients and potentially reducing the need for expensive interventions. The success of WISDOM could have far-reaching implications for chronic disease management and the use of health data for research and innovation, benefiting patients, healthcare providers, and society.
EMSP Role:
EMSP is actively involved in various work packages (WPs) of the project, contributing significantly to its objectives:
WP1 – Barriers to Healthcare Data Integration and Access: EMSP collaborates in investigating factors crucial for nurturing trust in computational models among patients and clinicians through surveys and interviews. This task is interlinked with WP5 and WP6, focusing on understanding clinicians’ views on AI implementation in clinics and patients’ perspectives on AI ethics in patient care, including privacy, information rights, and non-discrimination.
WP6 – Innovation, Communication, Dissemination (EMSP Co-Lead): EMSP’ MS Community Advisory Board plays a pivotal role in all stages of the project, from ideation to prototype development ensuring the patients’ voice is considered in all the stages. EMSP is involved in development and implementation of the communication and dissemination plan, which includes managing social media channels, posting publications, promoting the project successes during the international conferences, issuing press releases, etc. Furthermore, EMSP will engage stakeholders, organising workshops, and disseminating targeted newsletters and policy briefs to share project findings and understand the needs of healthcare providers, researchers, caregivers, and patient organisations.
Who is involved:
Eight European universities, leaders in the medical and analytical field, three SMEs, one research institute and one company, at the forefront of clinical AI implementation, data infrastructure, and security, and EMSP formed the consortium WISDOM.
- Karoliska Institutet, (“KI”) Department of Clinical Neuroscience, Stockholm, Sweden (the “Coordinator”)
- University of Helsinki, (UH), Finland
- Technical University of Munich (TUM-MED), Germany
- Copenhagen University (UCPH), Denmark
- Veilai OY (VEIL), Finland
- YoutHealth AB (YH), Sweden
- University of Southern Denmark (SDU), Denmark
- Centre for Genomic Regulation (CRD), Spain
- Decode Genetics INC. (deCODE), Iceland
- University of Eastern Piedmont Amedeo Avogardo (UPO), Italy
- University of Tartu, (TARTU), Estonia
- European Multiple Sclerosis Platform (EMSP), Brussels, Belgium
- Karolinska Institutet Innovations AB, (KI-Innovations) Sweden
- The Chancellor Master and Scholars of the University of Cambridge (CU), UK
Duration: 5 years, starting from 1 December 2023.
Funding:

This project has received funding from the European Union’s Horizon Europe Research and Innovation Actions under grant no. 101137154 (WISDOM).
Want to know more?
 Your Account
Your Account
