
Engaging Expert Patients: MS CAB and EBV-MS Project Team Collaborate on Clinical Trial Design
12.07.2024On June 19, 2024, the Multiple Sclerosis Community Advisory Board (MS CAB) met with the EBV-MS project research team for a pivotal consultation. The objective of this meeting was to ensure meaningful engagement and involvement of expert patients in the ongoing EBV-MS clinical trials, specifically focusing on the design of Phase 2b in a patient-centric manner.
Facilitated by Rob Camp from EUPATI Spain and Patricia Moghames and Mohsharif Nasrulloeva from the European Multiple Sclerosis Platform (EMSP), the meeting brought together MS CAB members with the EBV-MS researchers Professor Gro Owren Nygaard and Professor Øivind Fredvik Grytten Torkildsen. The discussion centred around evaluating the risks and benefits of trial participation, addressing crucial patient information needs, and fostering a collaborative environment to enhance the trial’s design and implementation.
What is the MS CAB?
The MS CAB is a coalition of MS community activists and patient advocates from across Europe within the EMSP. The MS CAB members possess extensive knowledge in scientific and policy-related matters. Comprised of well-trained and informed patients and caregivers, through EMSP, the MS CAB collaborates with researchers, companies, and other stakeholders to enhance clinical trials and accelerate the development and availability of treatments and cures for MS.
By providing expert patient perspectives, identifying research priorities, and ensuring patient-centric trial designs, the MS CAB plays a pivotal role in advancing MS research and improving access to effective and affordable treatments for the MS community.

Figure 1. What is the MS CAB
What is the consultation process like?
Before the meeting with the MS CAB, the EBV-MS project researchers provided a concise overview of their research questions and expectations. Based on the briefing session conducted by EMSP with the CAB members prior to the meeting, the MS CAB provided preliminary feedback to the EBV-MS researchers and required more background information and reading materials in preparation for the consultation together.
The EMSP and the EBV-MS project teams collaboratively planned and coordinated the meeting. This meeting was an open, ongoing discussion, allowing the EBV-MS researchers to share their questions, while encouraging the MS CAB members to ask questions, raise their concerns, and provide open feedback simultaneously (Figure 2).
Following the meeting, EMSP developed a comprehensive report capturing the insights and recommendations discussed. This report, along with additional post-meeting feedback from MS CAB members, offered valuable patient-driven perspectives to guide the researchers in the design of their trial and the development of the consent and information sheet.
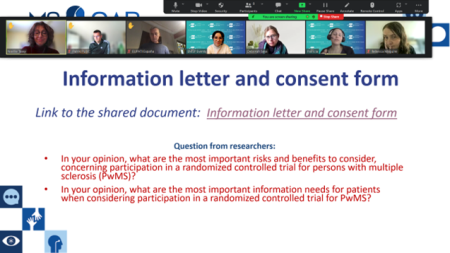
Figure 2. CAB members sharing their feedback and insights on study design and consenting process.
Why was it important for the EBV-MS researchers to consult with expert patients?
Consulting with expert patients when designing a clinical trial is crucial because it ensures that the study is aligned with the real-world needs and experiences of those living with the condition. Expert patients through MSCAB provided invaluable insights into the practical aspects of living with MS, including the challenges they face and their treatment priorities.
This patient-centered approach enhances the relevance and feasibility of the clinical trial, improves participant recruitment and retention, and ultimately leads to more meaningful and applicable outcomes.
By incorporating the perspectives of those directly affected, EBV-MS researchers, Professor Gro Owren Nygaard and Professor Øivind Fredvik Grytten Torkildsen can design more effective and compassionate protocols of the clinical trials, ending with more effective treatments that truly address the needs of the MS community. By involving the MS CAB the researchers gained valuable insights that would help design more patient-centered studies, leading to better outcomes and enhanced quality of life for those affected by MS.
What did the researchers and MS CAB members say after the meeting?
Through the post-meeting evaluation, the MS CAB members felt their perspectives were understood and valued by the researchers during the discussion, and they believed their input could significantly influence the research.
“There is a strong call for early and continued involvement of CAB members in the research process and document review.” – shared one MS CAB member.
The positive reception of the meeting’s structure and the collaborative atmosphere suggested a solid foundation for ongoing engagement and influence on the EBV-MS clinical trial.
As for one of the EBV-MS researchers,
“I was very impressed by the professionalism and insights from the consultation group. Their inputs and perspectives were instrumental in shaping and improving on the study design” – shared Professor Torkildsen.
Key takeaways from this MS CAB consultation
Researchers and people with MS highlighted the importance of early and continuous involvement of CAB members in research. The meeting underscored patient perspectives, ethical research considerations, and potential low-cost treatments.
The EBV-MS project team will further evaluate insights from the MS CAB to refine strategies and trial designs. Ongoing collaboration will keep the patient’s voice central, with researchers planning a follow-up meeting. Final findings will be transparently shared with the MS CAB and the broader community, guiding future research to align with MS patients’ needs.
This consultation demonstrates the value of incorporating patient perspectives to accelerate effective treatments for MS. Engaging the MS CAB ensures patient-centered outcomes, a practice that should become standard in research design.

This project has received funding from the European Union’s Horizon Europe Research and Innovation Actions under grant no. 101136991 (EBV-MS).
 Your Account
Your Account


