
Research and innovation: 13 things people with MS expect from N2B-patch
30.08.2019In 2017, an innovative project called N2B-patch (Nose-to-Brain-patch) was awarded a grant by the European Union’s H2020 programme for Research and Innovation. We embarked on this 4-year journey with a consortium of committed scientific experts and partners to support the development of a new device that would transform the landscape of drug delivery systems for people with multiple sclerosis (MS) and potentially for other diseases.
A survey gathered the perspective of over 300 people living with MS across Europe to better understand their expectations from this project. Their questions as well as other questions that might be important for the MS community were recently responded by the N2B-patch scientific team to help the MS community to better understand the efforts undertaken by the scientists.

Here are the 13 most important things people with MS want to know about this innovative EU funded research project.
1. The objectives of N2B-patch
We aim to develop an innovative technology for MS treatment by developing a ‘nose to brain’ delivery system which would reduce the use of injections and oral medicine. The direct transport route from the nose to the brain would offer a novel method for delivery of a broad variety of drugs directly into the central nervous system. The long-term objective is to improve treatment of MS patients.
2. An alternative for injections and oral medicine
Currently, nasal delivery is predominantly limited to nasal s prays or inhalation. The N2B-patch is a new administration technique and no clinical experiences are available at the moment. According to ear-nose-throat specialist, no pain or effect on smelling and tasting are expected. Nevertheless, a final statement cannot be made before more studies are performed in the field.
3. MS cure or improved treatments
N2B-patch seeks to achieve a more efficient way to deliver existing treatments to MS patients in a way that more drug reaches the affected regions of the central nervous system (CNS, brain and spinal cord) but the objective is not finding a cure for MS. However, using the new technique as a platform for drug delivery directly to the CNS may facilitate development of new drugs in the future. Direct effects in the CNS could potentially increase therapeutic efficacy of different drugs.

4. Administration of drugs on a less frequent basis
We are seeking to minimise the frequency of drug administration and to reduce the amount of medicine required for each dose. Our aim for the patch is to deliver a drug at least for one week or longer. This would help improve the quality of life of people with MS.
5. Any MS drug and use for other neurological diseases
The N2B-patch platform is being developed in a way that may allow the technology to be exploited for use with other medicines and for other neurological conditions.
6. Self-administration to avoid hospital visits?
Will the administration of drugs via the N2B-patch device require the intervention of healthcare professional or can the patient be able to manage the patch by themselves? The answer is yet to be concluded.
Most likely the patient will still require the drug to be administered by a trained healthcare professional. The first generation of N2B-patch shall be administered in specialized clinics or in the praxis of ear-nose-throat doctors that received a special training. Later generations of the patch might be suitable for self-administration.
7. Improving patient treatment adherence
It is a regulatory requirement to justify and prove the quality, safety and efficacy of the drug whilst at the same time demonstrating the clinical benefit, state of the art and performance of the device. The risk ratio/benefit must be justified, and patient adherence is also a fundamental aspect of the N2B-patch development.
This MS patient survey summary forms one small part of the data collection which is necessary to put the patient first and to understand patient expectations.
8. Expected side effects
N2B-patch is currently in a research phase and the aim is to achieve proof of concept. The involved scientists and technical experts are working intensively to generate data which is intended to demonstrate that this design concept is feasible and to prove the future potential for this technology. No human clinical trials are planned during the scope of this project; thus, side effects will not be known.
9. When will we be able to use the Nose-to-Brain-patch?
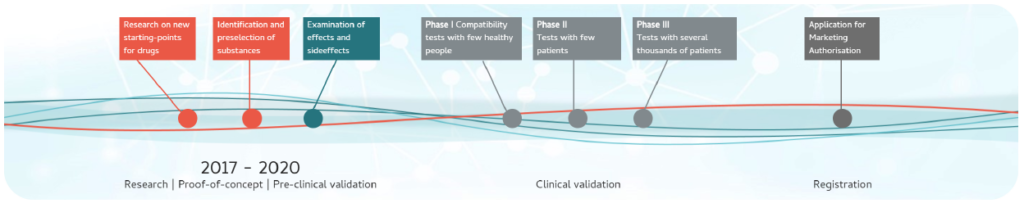
N2B-patch is numerous years away from regulatory approval and it is not anticipated that a regulatory submission would be made within the next 5 years.
This is because N2B-patch must go through a series of very large and expensive clinical trial phases to test whether it is safe and whether it works. Patient recruitment and retention will be quite challenging due to varying levels of disease progression and measuring clinical endpoints. MS patients and patient groups involvement will be absolutely essential to bringing this innovative drug delivery system to market.
10. The idea and motivations of scientists
The blood-brain barrier (BBB) is a physical barrier which separates the central nervous system (CNS) from general circulation. However, this barrier also limits direct access of different drugs like monoclonal antibodies or cytokines to the CNS. This fact limits the therapy of highly efficient drugs severely.
The N2B-patch research project represents a great challenge for the scientists and technical experts as they aim to overcome the BBB via bypassing drugs through nose directly to the brain.
11. Latest developments in MS research
Although modest in effect, new therapies for chronic progressive disease (primary and secondary progressive disease; PPMS and SPMS) are an important milestone in drug development. Also approaches to try to tailor the benefit risk profile of the drugs to individual patients are moving forward. This is particularly important since we have recently also seen unanticipated adverse drug reactions of already established drugs.
12. Why are patients and EMSP involved in the project?
It is of crucial importance to consult the patient community on their needs and expectations to ensure that the final product meets those. MS is a life-long disease where treatments and care are crucial to help maintain a productive and fulfilling life.
However, painful and uncomfortable drug delivery has an impact on the overall quality of life of MSers reducing their productivity. By working on this device together with the MS community and ensuring that their feedback is integrated along the way, the chances that it fits the expectations will be higher.
13. Why is the EU funding this project?
MS is the most important disease of the central nervous system affecting young and middle aged Europeans (about 2 to 3 times more common in women than in men). It leads to progressive invalidity in its chronic stage, a life with disabilities in a wheel chair and enormous socio-economic burden.
In Europe, the total costs of MS are about €14.6 billion euros as MS is a progressive disease with a poor prognosis.
The European Commission is aware that demyelinating diseases like MS are a burden for patient, their families and friends but also for the national health care systems as this disease concerns predominantly younger adults. Therefore, the EU supports and funds research and development in the field of demyelinating diseases to develop new therapies and rehabilitation, as well as innovative medical devices to improve the quality of life of people with MS and to reduce the socioeconomical burden.

Multiple Sclerosis (MS)
Multiple sclerosis (MS) is a complex neurological condition that affects nerves in the brain and spinal cord, causing a wide range of symptoms including problems with muscle movement, balance and vision. It is an autoimmune disease in which immune cells attack and destroy the protective myelin sheaths that surround nerve fibres, leading to neurological disturbances. Symptoms range from fatigue to severe mobility problems and disability.
 Your Account
Your Account


