EMSP at ECTRIMS 2016: Delivering for MS communities
21.09.2016EMSP’s team was present between 14 and 17 September at the Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS 2016), held in London, United Kingdom.
We were among 9,000 participants from almost 100 countries worldwide. And we took full advantage of this record attendance to promote our key deliverables for the MS communities in Europe and plan for the road ahead together with our partners and supporters.
Find below a report of EMSP’s headline activities at ECTRIMS.
Find treatment-related ECTRIMS news here.
MS Nurse Pro explained
ECTRIMS 2016 provided an excellent opportunity for the steering committee of MS Nurse Professional to shed further light on this growing project. At present, MS Nurse Pro is available in eight European languages and boasting more than 3,000 registered MS nurses.
In a series of short video interviews recorded and disseminated during the Congress, the project stakeholders talked about MS Nurse Pro‘s history, achievements and plans for the future.
Find two interviews below:
https://www.youtube.com/watch?v=HAkeDgI1t8w
https://www.youtube.com/watch?v=-OzwdC89guI
You can watch more MS Nurse Pro videos on our YouTube channel.
For more details on the project read our new MS Nurse Pro presentation brochure.
And to get a better feel of the magnitude of the ECTRIMS Congress watch a short presentation video:
https://www.youtube.com/watch?v=B-FCbV44sC8
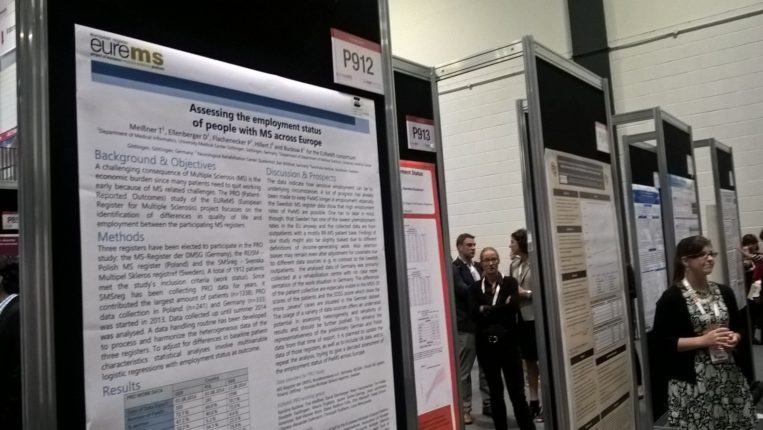
European Register for MS poster presentation
Our European Register for MS (EUReMS) project was successfully concluded in 2014 by pooling data from 12 MS registries across Europe and using this data to produce four landmark studies.
The EUReMS study Assessing the employment status of people with MS across Europe featured in the Congress’ poster exhibition.
Find the online version of the poster here.
Also, find more information on the EUReMS studies here.
EUReMS: next steps
EMSP met with EUReMS partners, the ‘Big MS Data group’ of five prominent European MS registries, US-based I Conquer MS initiative and industry supporters. Together, we discussed how to continue reaping the benefits of the winning formula of cross-border collaboration on MS registries.
EMSP’s latest proposal towards this goal is EuNetMuS, the European Network of Patient registries, Cohorts and Databases in Multiple Sclerosis.
This is a streamlined and re-positioned project on better exploitation of real world evidence data for research, regulatory work, health technology assessment and patient advocacy.
EMSP aims to reach an agreement between all EUReMS data collectors on standardised core data sets for clinical data, PRO data and socio-economic data.
This standardisation would allow the comparison and temporary pooling as well as centralised analysis of the aggregated data in a way which would answer vital questions. One such question is how to ensure the best possible safety of new MS therapies?
A recent simulation by the Newdigs think-tank showed a potential saving of up to 50 percent of the costs of authorizing a new drug through using patient registry data instead of the traditional clinical research organisation (CRO) data.
In conclusion, the simulation indicates that the combination between financial advantage and sustainability of patient registries can represent the difference between success and failure in such a venture.
Dropping the Mask: MS disclosure videos
EMSP was proud to launch two inspiring advocacy videos which are part of our Dropping the Mask campaign in support of MS disclosure.
The videos talk about the challenge of disclosing the MS diagnosis, in particular within an intimate relationship.
Find the first video below:
And the second on our YouTube channel.
The video production benefited from the invaluable help of our members and supporters. They enabled us to raise 6,000 euros.
Dropping the Mask is an initiative of EMSP’s Young People’s Network and demonstrates the desire of our young and vibrant MS community to combat stigma and promote patient empowerment.
EMSP and ECTRIMS strengthen collaboration
At the end of a successful bilateral meeting the ECTRIMS Board agreed to provide an official endorsement together with substantial financial support for EMSP’s MS Nurse Professional project. The funding will go towards the development of a sixth training module on MS rehabilitation.
This decision was taken on the basis of a Memorandum of Understanding (MoU) approved between EMSP and the ECTRIMS Board just days before the London Congress.
As the name suggests, the MoU aims to strengthen the ongoing collaboration between our two organisations – with a focus on speeding up progress in care and treatment for people with MS.

ECTRIMS, on the path to EMA recognition
EMSP brought together the ECTRIMS Board and representatives of the European Medicines Agency (EMA) for a fruitful meeting. This resulted in an agreement to start the registration process of ECTRIMS as an EMA-recognised MS organisation.
Once formalised, this decision will – among other things – allow ECTRIMS to be consulted by EMA in MS drugs market authorization processes.
The outcomes of this meeting are fully in line with EMSP’s commitment to provide the best possible treatment information and access for our MS communities.
Hot topics
The EMSP team attended a number of ECTRIMS 2016 sessions, presentations and debates reflecting strategic priority areas included in our Code of Good Pratice in MS. Among these priorities are patient empowerment, the provision of care and paediatric MS.
Find below a selection of topics, some intensely debated:
MS offspring: children of parents living with MS. Talking point: These children tend to get higher grades in basic school and similar educational level.
Paediatric MS: the needs of children living with MS
Clinical and social implications of aging with MS. Talking point: There are fewer MS-related mental health issues in older patients living with MS as they tend to be more familiar with this condition, despite generally carrying a higher degree of disability than young people with MS do.
A multidisciplinary perspective and patient activation programmes in MS management. Talking point: MS patients should be ‘activated’ to take control over their disease, and rehabilitation should be a part of routine clinical practice in the management of MS.
Burning debate: Should people with MS be included in trials for progressive MS? (pictured above) Talking point: The reasons why a significant proportion of people with MS are excluded – and could continue to be excluded – from clinical trials of disease-modifying treatments. Find the related Twitter coverage here.
MS Quiz
For the first time in EMSP’s long history of ECTRIMS participation we disseminated an MS quiz.
Find the online version of the quiz here.
We would like to thank all the offline and online participants and to announce the big winner: Tina Meissner (pictured below), an MS researcher from Germany and co-author of our EUReMS poster at ECTRIMS 2016.
Her prize: Tina will have a place reserved at our 2017 Annual Conference, to be held next May in Athens, Greece (find quiz poster below).

This MS quiz exercise was meant not so much to test the participants’ MS knowledge – most were specialists in the field – but to encourage them to learn more about EMSP’s projects and activities.
EMSP on social media
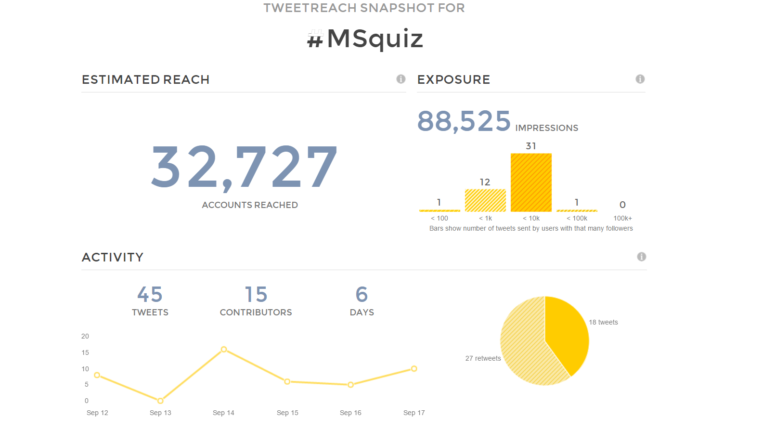
Our #ECTRIMS2016 MS Quiz was warmly received by Twitter followers, reaching over 32,000 accounts (print screen of related Twitter analysis below).
EMSP’s Facebook profile also received a significant boost, for example by reaching almost 7,000 people with the promotion of the two Dropping the Mask videos.
We were also active on LinkedIn during ECTRIMS.
We always appreciate new followers and subscribers!
ECTRIMS 2016 in numbers
Active participants: over 8,300
Total participants: over 9,300
Countries of origin: 96
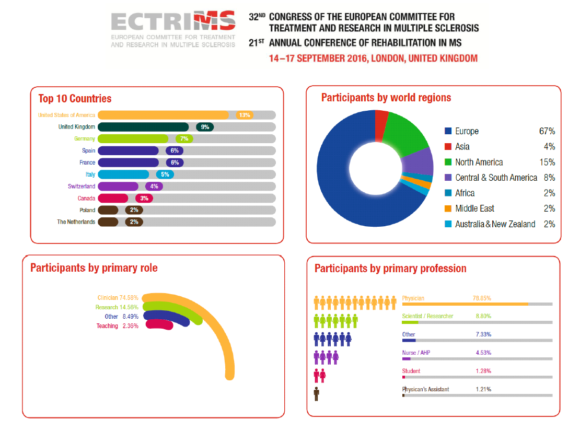
Submitted study abstracts: over 2,000
Looking forward to ECTRIMS 2017: in Paris, France!
 Your Account
Your Account